
SPEVIGO IV
(intravenous infusion)
was evaluated in the
Effisayil® 1 trial1,2
EFFISAYIL 1 trial was a multicenter, randomized, double-blind, placebo-controlled trial of SPEVIGO IV in patients with GPP experiencing a flare1
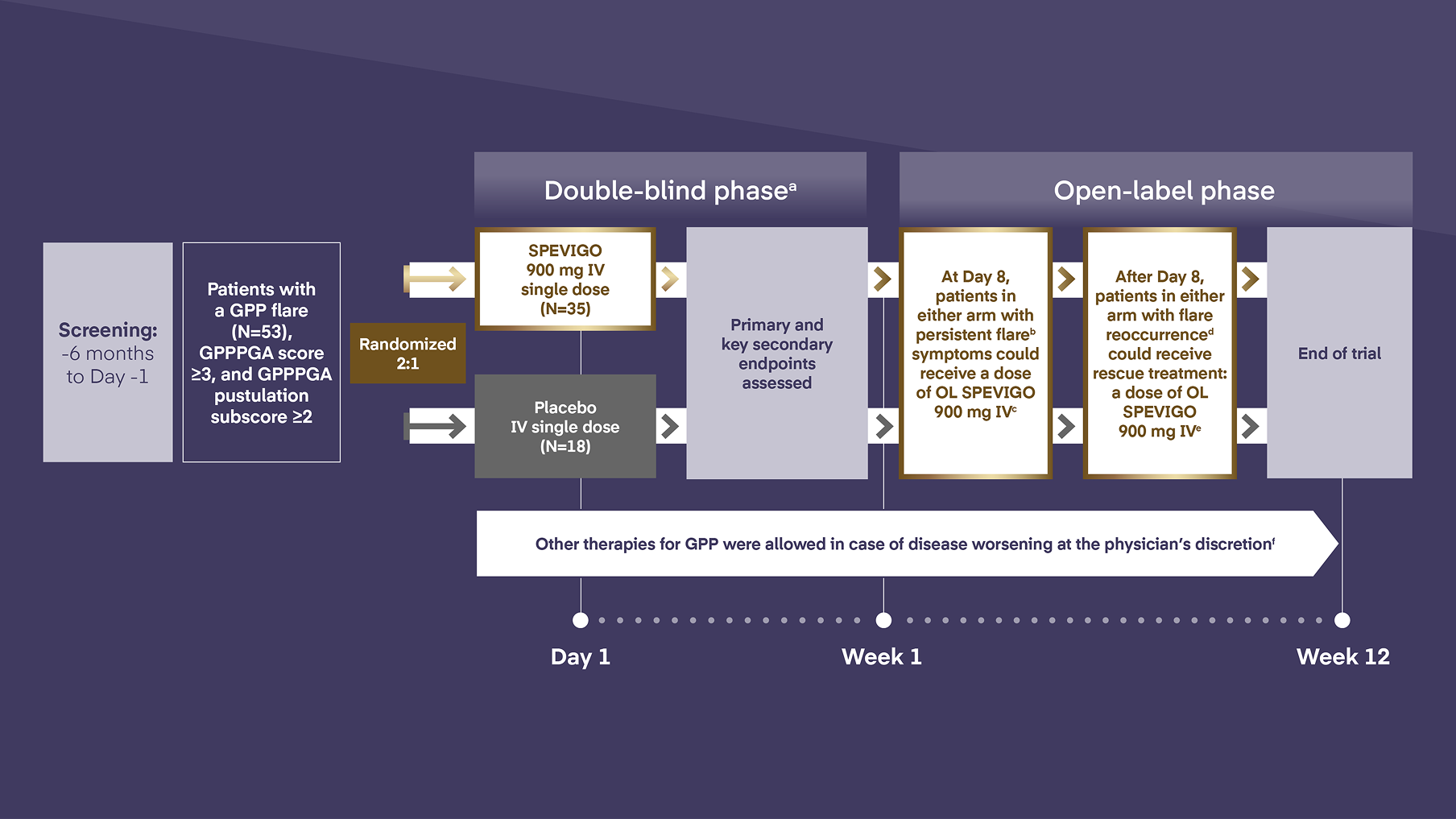
-
a
Patients must have discontinued biologics, retinoids, methotrexate, and/or cyclosporine before receiving their first dose of SPEVIGO IV or placebo.1
-
b
Persistent flare defined as ≥2 GPPPGA total score and ≥2 GPPPGA pustulation subscore.1
-
c
Patients who received other medications for GPP during Week 1 were not eligible for SPEVIGO IV at Day 8.3
-
d
Defined as a ≥2-point increase in GPPPGA pustulation subscore ≥2 after achieving clinical response (GPPPGA total score of 0 or 1).1
-
e
After Day 8, patients with a new flare (defined as ≥2-point increase in GPPPGA total score and pustulation subscore) could receive OL SPEVIGO IV if a GPPPGA total score of 0 or 1 had been reached with SPEVIGO IV or placebo before.1
-
f
At the end of Week 1, 2 patients on SPEVIGO IV and 1 patient on placebo had received other treatment for GPP. From Day 8 to the end of the trial, 4 patients in each arm received other treatment for GPP.1
-
GPP, generalized pustular psoriasis; GPPPGA, Generalized Pustular Psoriasis Physician Global Assessment; IV, intravenous infusion; OL, open-label.
Endpoint summary1,2
| Primary endpoint |
|---|
| Proportion of patients with GPPPGA pustulation subscore of 0 at Week 1 |
| Key secondary endpoint |
|---|
| Proportion of patients with GPPPGA total score of 0 or 1 at Week 1 |
| Additional secondary endpoints |
|---|
|
FACIT, Functional Assessment of Chronic Illness Therapy; GPPASI, Psoriasis Area and Severity Index for Generalized Pustular Psoriasis; GPPPGA, Generalized Pustular Psoriasis Physician Global Assessment; PSS, Psoriasis Symptom Scale; VAS, Visual Analogue Scale.
In EFFISAYIL 1, SPEVIGO IV was evaluated across various demographics and baseline characteristics1
| Characteristics | SPEVIGO IV (N=35) | Placebo (N=18) |
|---|---|---|
| Mean age (SD), years | 43.2 (12.1) | 42.6 (8.4) |
| Mean weight (SD), kg | 73.7 (24.0) | 68.8 (26.6) |
| Female, n (%) | 21 (60) | 15 (83) |
| Raceg
Asian, n (%) White, n (%) | 16 (46) 19 (54) | 13 (72) 5 (28) |
| Baseline CRP, n (%)h
≥3 mg/L to <70 mg/L ≥70 mg/L | 20 (58.8) 11 (31.4) | 12 (66.7) 4 (22.2) |
| GPPPGA total score, n (%) 3 4 | 28 (80) 7 (20) | 15 (83) 3 (17) |
| GPPPGA pustulation subscore, n (%) 2 3 4 | 6 (17) 16 (46) 13 (37) | 5 (28) 7 (39) 6 (33) |
This study did not include a sufficient number of subjects to determine if there are differences in response according to the various baseline demographics and characteristics.2
-
g Race was reported by the patient.1
-
h A total of 52 patients were included: 34 in the SPEVIGO arm and 18 in the placebo arm. Five patients had missing values at baseline.1
-
CRP, C-reactive protein; IV, intravenous infusion; SD, standard deviation.
Patients aged 18 to 75 years with Generalized Pustular Psoriasis (GPP), as defined by ERASPEN:
Primary, sterile, macroscopically visible pustules on non-acral skin (excluding cases in which pustulation is restricted to psoriatic plaques)4
With or without systemic inflammation, with or without plaque psoriasis; can be either a relapsing condition (>1 episode) or a persistent condition (>3 months)
Evidence (or previous evidence) of systemic symptoms:
• Fever
• Asthenia
• Myalgia
• Elevated C-reactive protein level
• Leukocytosis with peripheral blood neutrophilia (above ULN)
ERASPEN, European Rare and Severe Psoriasis Expert Network; ULN, upper limit of normal.
Patients were excluded if they presented with:
SAPHO syndrome
Plaque psoriasis without pustules or with pustules restricted to psoriatic plaques
Drug-triggered AGEP
Immediate, life-threatening GPP flare or required intensive care treatment
Dose escalation of their maintenance treatment with cyclosporine, retinoids, or methotrexate within 2 weeks prior to randomization
Treatment with any drug, including biologics and systemic drugs, considered likely to interfere with the safe conduct of the study or any prior exposure to an IL-36R inhibitor
AGEP, acute generalized exanthematous pustulosis; IL-36R, interleukin-36 receptor; SAPHO, synovitis-acne-pustulosis-hyperostosis-osteitis.
The Generalized Pustular Psoriasis Physician Global Assessment (GPPPGA) is used to evaluate GPP symptoms and treatment efficacy4,5
Flare controli includes clearance of pustules, erythema, and scaling4,5

Burden AD, et al. Br J Dermatol. 2023;189(1):138–140.5
The GPPPGA total score is the calculated average of the composite scores for pustules, erythema, and scaling skin.3 Each of these components is scored individually from 0 (clear) to 4 (severe).3
-
i
Flare control defined as GPPPGA total score ≤1 and was reported for the 12-week study period.1
-
j
To receive a score of 0 or 1, the patient should be afebrile, in addition to skin presentation requirements.3
-
GPP, generalized pustular psoriasis; GPPPGA, Generalized Pustular Psoriasis Physician Global Assessment.
References
-
Bachelez H, Choon SE, Marrakchi S, et al; for the EFFISAYIL 1 Trial Investigators. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385(26):2431–2440.
-
SPEVIGO [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. May 2025.
-
Choon SE, Lebwohl MG, Marrakchi S, et al. Study protocol of the global EFFISAYIL 1 phase II, multicentre, randomised, double-blind, placebo-controlled trial of spesolimab in patients with generalized pustular psoriasis presenting with an acute flare. BMJ Open. 2021;11(3):e043666.
-
Navarini AA, Burden AD, Capon F, et al; for the ERASPEN Network. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1792–1799.
-
Burden AD, Bachelez H, Choon SE, et al. The Generalized Pustular Psoriasis Physician Global Assessment (GPPPGA) score: online assessment and validation study of a specific measure of GPP disease activity. Br J Dermatol. 2023;189(1):138–140.
SPEVIGO is indicated for the treatment of generalized pustular psoriasis (GPP) in adults and pediatric patients 12 years of age and older and weighing at least 40 kg.
CONTRAINDICATIONS
SPEVIGO is contraindicated in patients with severe or life-threatening hypersensitivity to spesolimab-sbzo or to any of the excipients in SPEVIGO. Reported hypersensitivity reactions have included drug reaction with eosinophilia and systemic symptoms (DRESS) and anaphylaxis.
WARNINGS AND PRECAUTIONS
Infections: SPEVIGO may increase the risk of infections. In patients with a chronic infection or a history of recurrent infection, consider the potential risks and expected clinical benefits of treatment prior to prescribing SPEVIGO. Treatment with SPEVIGO is not recommended in patients with any clinically important active infection until the infection resolves or is adequately treated. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur during or after treatment with SPEVIGO. If a patient develops a clinically important active infection, discontinue SPEVIGO therapy until the infection resolves or is adequately treated.
Risk of Tuberculosis: Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with SPEVIGO. Avoid use of SPEVIGO in patients with active TB infection. Consider initiating anti-TB therapy prior to initiating SPEVIGO in patients with latent TB or a history of TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after SPEVIGO treatment.
Hypersensitivity and Infusion-Related Reactions:
- Serious hypersensitivity reactions, including anaphylaxis and delayed reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS), have been reported during and following administration of SPEVIGO. These reactions can occur with the first dose or subsequent doses.
- SPEVIGO is contraindicated in patients with severe or life-threatening hypersensitivity to spesolimab-sbzo or to any of the excipients in SPEVIGO. If a patient develops signs of anaphylaxis or other serious hypersensitivity, discontinue SPEVIGO immediately and initiate appropriate treatment.
- If a patient develops mild or moderate hypersensitivity during an intravenous infusion or other infusion-related reactions, stop SPEVIGO infusion and consider appropriate medical therapy (eg, systemic antihistamines and/or corticosteroids). Upon resolution of the reaction, the infusion may be restarted at a slower infusion rate with gradual increase to complete the infusion.
Vaccinations: Prior to initiating SPEVIGO for treatment of GPP, complete all age-appropriate vaccinations according to current immunization guidelines. Avoid use of live vaccines in patients during and for at least 16 weeks after treatment with SPEVIGO. No specific studies have been conducted in SPEVIGO-treated patients who have recently received live viral or live bacterial vaccines.
ADVERSE REACTIONS
Intravenous SPEVIGO for Treatment of GPP Flare (Study Effisayil-1): Most common adverse reactions reported in ≥5% of patients treated with SPEVIGO in the clinical trial were asthenia and fatigue, headache, nausea, pruritus and prurigo, infusion site hematoma and bruising, and urinary tract infection (UTI).
Specific Adverse Reactions
Infections: The most frequent adverse reactions that occurred in subjects treated with intravenous SPEVIGO were infections. During the 1-week placebo-controlled period in Study Effisayil-1, infections were reported in 14% of subjects treated with SPEVIGO compared with 6% of subjects treated with placebo. Serious infection (UTI) was reported in 1 subject (3%) in the SPEVIGO group and no subjects in the placebo group. Infections observed through Week 1 in Study Effisayil-1 in subjects treated with SPEVIGO were mild (29%) to moderate (71%).
Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): Two cases of DRESS were reported in Study Effisayil-1 in subjects with GPP who were treated with intravenous SPEVIGO. RegiSCAR DRESS validation scoring (with the following categories: “no,” “possible,” “probable,” or “definite” DRESS) was applied to the reported cases. Reported cases were assessed as “no DRESS” and “possible DRESS.”
Subcutaneous SPEVIGO for Treatment of GPP When Not Experiencing a Flare (Study Effisayil-2): Regarding the exposure-adjusted incidence rates for subjects on randomized treatment prior to receiving rescue treatment for flare or completing trial without a flare, the rate per 100-patient years for injection site reaction (including erythema, pain, swelling, induration, urticaria, and warmth at the injection site) was 31.6 for the subcutaneous SPEVIGO cohort (600 mg loading dose followed by 300 mg every 4 weeks) vs 12.7 for the placebo cohort. The rate per 100-patient years for UTI was 18 for SPEVIGO vs 0 for placebo. The rate per 100-patient years for pruritus was 8.8 for SPEVIGO vs 0 for placebo. The rate per 100-patient years for arthralgia was 13.3 for SPEVIGO vs 6 for the placebo cohort. There were 3 subjects who discontinued subcutaneous SPEVIGO due to treatment-emergent adverse events of psoriasis compared to no subjects in the placebo cohort who discontinued placebo for any treatment-emergent adverse event.
Safety in Study Effisayil-2 After Flare: In Effisayil-2, subjects who experienced a GPP flare and received at least one dose of an open-label single intravenous 900 mg dose of SPEVIGO were treated with open-label subcutaneous SPEVIGO 300 mg. These subjects (n=19) received subcutaneous dosing at every 12 weeks, which could have been increased to every 4 weeks based on GPPPGA total score or pustulation subscore increased by ≥1 from any previous open-label maintenance visit. The reported safety profile of open-label subcutaneous SPEVIGO use after treatment of GPP flare with open-label intravenous SPEVIGO use was consistent with the safety profiles of use of SPEVIGO from Trial Effisayil-1 and randomized controlled data from Trial Effisayil-2.
Clinical Development of Spesolimab-sbzo
Guillain-Barre Syndrome (GBS): Among approximately 835 subjects exposed to spesolimab-sbzo during clinical development, GBS was reported in 3 subjects who received various doses of spesolimab-sbzo via various methods of administration in clinical trials for unapproved indications.
SPECIFIC POPULATIONS
Pediatric Use: The safety and effectiveness of SPEVIGO for the treatment of GPP have been established in pediatric patients 12 years of age and older and weighing at least 40 kg. Use of SPEVIGO for this indication is supported by data from a randomized, placebo-controlled study, which included 6 pediatric subjects 14 to 17 years of age with a history of GPP treated with subcutaneous SPEVIGO (Study Effisayil-2), and evidence from an adequate and well-controlled study of intravenous SPEVIGO in adults with GPP (Study Effisayil-1), with additional pharmacokinetic analyses showing similar drug exposure levels in adults and pediatric subjects 12 years of age and older and weighing 40 kg or more. The safety and effectiveness of SPEVIGO in pediatric patients younger than 12 years of age or in pediatric patients weighing less than 40 kg have not been established.
CL-SPG-100006 10.23.2025
Please see SPEVIGO Prescribing Information, including Medication Guide.


